out of the MILLIONS of people who've had it...
And we sent 18 yo to die for 20 years when 2500 died.... but it was too much to ask to wear a mask and get vaccinated...merica!
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
out of the MILLIONS of people who've had it...
You probably should read up on how the vaccination graphs Mr. Topol (NIH funded, captured doc) presents and why the data is flawed. But of course since you're Phenomenally Stupid, you'll believe everything thrown your way. CDC data is incomplete and assumed vax status if for whatever reason a person wasn't verified (each state/hospital is different) or didn't have proper dosing of clot shots in timeframe needed, and no proper vax data from 20 states. But yes keep touting data from the CDC who just so happened to have a "systems upgrade" so they could reclassify excess deaths because they have been going off the charts. Same thing happened with the DOD data "reclassify". Ah but don't worry if Trump was in office and his admin did these things you'd believe otherwise.
Vaccination status: A person vaccinated with a primary series had SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected ≥14 days after verifiably completing the primary series of an FDA-authorized or approved COVID-19 vaccine. An unvaccinated person had SARS-CoV-2 RNA or antigen detected on a respiratory specimen and has not been verified to have received COVID-19 vaccine. Excluded were partially vaccinated people who received at least one FDA-authorized vaccine dose but did not complete a primary series ≥14 days before collection of a specimen where SARS-CoV-2 RNA or antigen was detected.
Additional or booster dose: A person vaccinated with a primary series and an additional or booster dose had SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected ≥14 days after receipt of an additional or booster dose of any COVID-19 vaccine on or after August 13, 2021. For people ages 18 years and older, data are graphed starting the week including September 24, 2021, when a COVID-19 booster dose was first recommended by CDC for adults 65+ years old and people in certain populations and high risk occupational and institutional settings. For people ages 12-17 years, data are graphed starting the week of December 26, 2021, 2 weeks after the first recommendation for a booster dose for adolescents ages 16-17 years. For people ages 5-11 years, data are included starting the week of June 5, 2022, 2 weeks after the first recommendation for a booster dose for children aged 5-11 years. For people ages 50 years and older, data on second booster doses are graphed starting the week including March 29, 2022, when the recommendation was made for second boosters. Vertical lines represent dates when changes occurred in U.S. policy for COVID-19 vaccination (details provided above). Reporting is by primary series vaccine type rather than additional or booster dose vaccine type. The booster dose vaccine type may be different than the primary series vaccine type.
Deaths: A COVID-19–associated death occurred in a person with a documented COVID-19 diagnosis who died; health department staff reviewed to make a determination using vital records, public health investigation, or other data sources. Rates of COVID-19 deaths by vaccination status are reported based on when the patient was tested for COVID-19, not the date they died. Deaths usually occur up to 30 days after COVID-19 diagnosis.
Participating jurisdictions: Currently, these 31 health departments that regularly link their case surveillance to immunization information system data are included in these incidence rate estimates: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, District of Columbia, Florida, Georgia, Idaho, Indiana, Kansas, Kentucky, Louisiana, Massachusetts, Michigan, Minnesota, Nebraska, New Jersey, New Mexico, New York, New York City (New York), North Carolina, Philadelphia (Pennsylvania), Rhode Island, South Dakota, Tennessee, Texas, Utah, Washington and West Virginia; 30 jurisdictions also report deaths among vaccinated and unvaccinated people. These jurisdictions represent 72% of the total U.S. population and all ten of the Health and Human Services Regions. Data on cases among people who received additional or booster doses were reported from 30 jurisdictions; 29 jurisdictions also reported data on deaths among people who received one or more additional booster dose; 26 jurisdictions reported cases among people who received two or more additional or booster doses; and 25 jurisdictions reported deaths among people who received two or more additional or booster doses. This list will be updated as more jurisdictions participate.
Incidence rate estimates: Weekly age-specific incidence rates by vaccination status were calculated as the number of cases or deaths divided by the number of vaccinated with a primary series, overall or with/without a booster dose (cumulative) or unvaccinated (obtained by subtracting the cumulative number of people vaccinated with a primary series and current estimates of partially vaccinated people from the 2019 U.S. intercensal population estimates) and multiplied by 100,000. Overall incidence rates were age-standardized using the 2000 U.S. Census standard population. All rates are plotted by positive specimen collection date to reflect when incident infections occurred. For the primary series analysis, age-standardized rates include ages 12 years and older from April 4, 2021 through December 4, 2021, and include ages 5 years and older from December 5, 2021 onwards. For the booster dose analysis, age-standardized rates include ages 18 years and older from September 19, 2021 through December 25, 2021, ages 12 years and older from December 26, 2021, and ages 5 years and older from June 5, 2022 onwards. Small numbers could contribute to less precision when calculating death rates among some groups.
Continuity correction: A continuity correction has been applied to the denominators by capping the percent population coverage at 95%. To do this, we assumed that at least 5% of each age group would always be unvaccinated in each jurisdiction. Adding this correction ensures that there is always a reasonable denominator for the unvaccinated population that would prevent incidence and death rates from growing unrealistically large due to potential overestimates of vaccination coverage.
Incidence rate ratios (IRRs): IRRs for the past one month were calculated by dividing the average weekly incidence rates among unvaccinated people by that among people vaccinated with a primary series either overall or with a booster dose.

Yeah everyone, we know we tried to force you into the shots just a few months ago, but we’ve now learned they’re poisonous, but trust us when we try to force you to get the next round…
What are the long term effects from the mRNA vaccines? Could we discover 10 years from now multiple injections leads to kidney failure, sexual dysfunction, cancer? How many decades later did we learn baby powder was carcinogenic?
THERE’S NO TIME!! It’s a pandemic, damnit! We can deal with stuff like safety & efficacy & ADE later....we need everyone injected NOW, ya damned fool.
It's actually >1 million dead, spud.out of the MILLIONS of people who've had it...
or just stop pretending like we all aren't going to die of something. if trying to cure diseases is what leads to the creation of new viruses... maybe it is time to stop.i guess we should stop trying to cure diseases.
Oh, it's okay. Only tens of thousands of people have died.
or just stop pretending like we all aren't going to die of something. if trying to cure diseases is what leads to the creation of new viruses... maybe it is time to stop.
Because the Biden administration has pushed for a fall booster campaign to begin in September, the mRNA vaccine-makers Pfizer-BioNTech and Moderna have only had time to test the reformulated shots in mice, not people. That means the Food and Drug Administration is relying on the mice trial data — plus human trial results from a similar vaccine that targets the original omicron strain, called BA.1 — to evaluate the new shots, according to a recent tweet from the FDA commissioner, Dr. Robert Califf.

Basing your entire news story premise on a "tweet" is not a terribly sound strategy.
NEW YORK & MAINZ, Germany--(BUSINESS WIRE)--Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced positive data evaluating the safety, tolerability, and immunogenicity of two Omicron-adapted COVID-19 vaccine candidates: one monovalent and the other bivalent, a combination of the Pfizer-BioNTech COVID-19 Vaccine and a vaccine candidate targeting the spike protein of the Omicron BA.1 variant of concern. Data from the Phase 2/3 trial found that a booster dose of both Omicron-adapted vaccine candidates elicited a substantially higher immune response against Omicron BA.1 as compared to the companies’ current COVID-19 vaccine. The robust immune response was seen across two investigational dose levels, 30 µg and 60 µg.
The Omicron adapted vaccine candidates (30 µg and 60 µg) studied in the Phase 2/3 trial in 1,234 participants 56 years of age and older elicited substantially higher neutralizing antibody responses against Omicron BA.1 when compared to the companies’ current COVID-19 vaccine. The pre-specified criterion for superiority was measured by the ratio of neutralizing geometric mean titers (GMR) with the lower bound of the 95% confidence interval >1. The geometric mean ratios (GMRs) for the monovalent 30 µg and 60 µg vaccines compared to the current COVID-19 vaccine were 2.23 (95% CI: 1.65, 3.00) and 3.15 (95% CI: 2.38, 4.16), respectively. The GMRs for the bivalent 30 µg and 60 µg vaccines compared to the current COVID-19 vaccine were 1.56 (95% CI: 1.17, 2.08) and 1.97 (95% CI: 1.45, 2.68), respectively. The monovalent Omicron-adapted vaccine 30 µg and 60 µg achieved a lower bound 95% confidence interval for GMR of >1.5, consistent with the regulatory requirement of super superiority. Demonstration of superiority against Omicron and safety are regulatory requirements for potential emergency use authorization of a variant-adapted vaccine.
Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron | Pfizer
Omicron-adapted monovalent candidate given as a fourth booster dose elicited a 13.5 and 19.6-fold increase in neutralizing geometric titers against Omicron BA.1 at 30 µg and 60 µg dose levels; bivalent vaccine candidate exhibited a 9.1 and 10.9-fold increase against Omicron Geometric mean ratios...www.pfizer.com

Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced positive data evaluating the safety, tolerability, and immunogenicity of two Omwww.businesswire.com
And that release was back in JUNE, when those clinical trials were completed IN HUMANS.

You are absolutely correct and this board is full of people who are narrative pushers.Neither the article he got that chart from (https://www.ft.com/content/26e0731f-15c4-4f5a-b2dc-fd8591a02aec) nor the study cited within (https://www.nature.com/articles/s41586-022-04569-5) even considered the possibility of ADE on brain function and/or damage. Ideally, there should have been four groups studied: never infected jabbed/unjabbed and infected jabbed/unjabbed.
There’s a reason Drs. Fauci, Hotez, Offit, etc., all were issuing extreme caution in the spring/summer of 2020 when it came to rushing mrna products into production and implementation
Ah yes good ole Riley the nitwitted imbecile. Poor whittle baby can't handle being proven how dumb he is....Look at who has banded together Pinehawk, KFsdisciple, your_master5 and Bill Doak - BWAAAAAHAHAHAHA
A band of idiots. It would be great if none of you get the vaccine, or buckle your seat belts, or come in from a lightning storm, or turn off the stove, or....
What a bunch of gullible dumb ****s.
You are literally making things up out of thin air now. JFC you're a real moron.So you do care about? Lol come tell everyone how a RCT on masking
No it does... it'd like a 90+ page paper and discusses it in quite detail.
The Danish study quite successfully proves masking would reduce ~3.3 million infections and if we say 1% get sever disease/death it's pretty clear wearing a simple mask that causes no harm in the US alone could have prevented 33k bad outcomes...
Yeah didn't expect anything of substance from your dumbass. Keep with the gifs that's more your territory.
Because neither worked buffoon.And we sent 18 yo to die for 20 years when 2500 died.... but it was too much to ask to wear a mask and get vaccinated...merica!
Ah yes let's release an updated version the EUA jab that will be for an outdated virus. Good job!Basing your entire news story premise on a "tweet" is not a terribly sound strategy.
NEW YORK & MAINZ, Germany--(BUSINESS WIRE)--Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced positive data evaluating the safety, tolerability, and immunogenicity of two Omicron-adapted COVID-19 vaccine candidates: one monovalent and the other bivalent, a combination of the Pfizer-BioNTech COVID-19 Vaccine and a vaccine candidate targeting the spike protein of the Omicron BA.1 variant of concern. Data from the Phase 2/3 trial found that a booster dose of both Omicron-adapted vaccine candidates elicited a substantially higher immune response against Omicron BA.1 as compared to the companies’ current COVID-19 vaccine. The robust immune response was seen across two investigational dose levels, 30 µg and 60 µg.
The Omicron adapted vaccine candidates (30 µg and 60 µg) studied in the Phase 2/3 trial in 1,234 participants 56 years of age and older elicited substantially higher neutralizing antibody responses against Omicron BA.1 when compared to the companies’ current COVID-19 vaccine. The pre-specified criterion for superiority was measured by the ratio of neutralizing geometric mean titers (GMR) with the lower bound of the 95% confidence interval >1. The geometric mean ratios (GMRs) for the monovalent 30 µg and 60 µg vaccines compared to the current COVID-19 vaccine were 2.23 (95% CI: 1.65, 3.00) and 3.15 (95% CI: 2.38, 4.16), respectively. The GMRs for the bivalent 30 µg and 60 µg vaccines compared to the current COVID-19 vaccine were 1.56 (95% CI: 1.17, 2.08) and 1.97 (95% CI: 1.45, 2.68), respectively. The monovalent Omicron-adapted vaccine 30 µg and 60 µg achieved a lower bound 95% confidence interval for GMR of >1.5, consistent with the regulatory requirement of super superiority. Demonstration of superiority against Omicron and safety are regulatory requirements for potential emergency use authorization of a variant-adapted vaccine.
Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron | Pfizer
Omicron-adapted monovalent candidate given as a fourth booster dose elicited a 13.5 and 19.6-fold increase in neutralizing geometric titers against Omicron BA.1 at 30 µg and 60 µg dose levels; bivalent vaccine candidate exhibited a 9.1 and 10.9-fold increase against Omicron Geometric mean ratios...www.pfizer.com

Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced positive data evaluating the safety, tolerability, and immunogenicity of two Omwww.businesswire.com
And that release was back in JUNE, when those clinical trials were completed IN HUMANS.
You are literally making things up out of thin air now. JFC you're a real moron.
Let me ask you this, if you were to remove asbestos would you wear a cloth mask? Surgical mask? N95?
Because neither worked buffoon.

The world’s top scientists and doctors on the off topic board disagree with you.
Before We Push the New Omicron Vaccine, Let’s See The Data
The White House is pushing Americans hard to take a novel Covid vaccine before the studies are complete.sensiblemed.substack.com
“One frustrated CDC official told me the vaccines are so ineffective in young children it wouldn’t matter if you, “inject them with it or squirt it in their face.” Maybe that’s why after a month of pushing Covid vaccination for children under five, only 3% of them got the jab.”

Before We Push the New Omicron Vaccine, Let’s See The Data
The White House is pushing Americans hard to take a novel Covid vaccine before the studies are complete.sensiblemed.substack.com
“One frustrated CDC official told me the vaccines are so ineffective in young children it wouldn’t matter if you, “inject them with it or squirt it in their face.” Maybe that’s why after a month of pushing Covid vaccination for children under five, only 3% of them got the jab.”
Well if all 8 mice were fine, then by all means let’s get 330 million people jabbed right away!!!Inflation is even hampering our research facilities now; eight mice. EIGHT!! Times are tough all over....
In the study, eight mice that were given the BA.5 booster dose about 100 days after receiving two doses of Pfizer’s original vaccine generated an immune response.
“To rely only on mouse data (for authorization) would be unprecedented in my knowledge and would certainly raise eyebrows,” said John Moore, a vaccine and virology expert at Weill Cornell Medicine in New York. “It doesn’t mimic the human situation,” where many people were vaccinated more than a year ago and have since been boosted.

Pfizer-BioNTech submits new COVID vaccine booster targeting BA.5 to the FDA for authorization
Pfizer and BioNTech have submitted their new COVID-19 booster that targets the omicron subvariant BA.5 to the FDA for emergency use authorization.www.usatoday.com
Inflation is even hampering our research facilities now; eight mice. EIGHT!!
Are flu vaccines new? Are they a first time used technology for vaccines?>1200 people were already tested, bud. In a Phase 2/3 trial
Which is 1200 MORE than are every tested with seasonal flu vaccines.
The numbers aren't there. You extrapolated the results using your own model. Every other RCT done on mask pre-covid shows it doesn't work. Danish/Bangladesh study shows they don't work (statistically insignificant). You keep doing an awesome job of being a complete fool.Nope you are... the numbers are quite clearly there and the Danish study clearly demonstrates it would have prevented 3.3 million infections in the US. Show us your amazing mask rct that works and not observational.
What dies asbestos have to do with covid?? You stupidity is quite amazing.
I’m mostly just a lurker but you really standout as thought challenged.Because the Biden administration has pushed for a fall booster campaign to begin in September, the mRNA vaccine-makers Pfizer-BioNTech and Moderna have only had time to test the reformulated shots in mice, not people. That means the Food and Drug Administration is relying on the mice trial data — plus human trial results from a similar vaccine that targets the original omicron strain, called BA.1 — to evaluate the new shots, according to a recent tweet from the FDA commissioner, Dr. Robert Califf.
That could be a potentially risky bet, experts say, if the shots don’t work as well as hoped.
Federal health officials hope that the new vaccines will provide stronger protection over the existing booster shots, which still target the original coronavirus strain. But the lack of data in humans means officials likely won’t know how much better the new shots are — if at all — until the fall booster campaign is well underway.
The FDA’s decision to move forward without data from human trials is a gamble, experts say, threatening to further lower public trust in the vaccines should the new boosters not work as intended.
Clinical studies in humans aren't required for the approval of seasonal influenza vaccines, even when they're reformulated for strain changes, said Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief.
Still, the flu vaccine isn’t a fair comparison, said Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia.
The FDA’s policy on influenza shots is based on decades of experiences with strain changes where the flu vaccines behaved generally in the same way. The U.S. is still on its first iteration of the Covid vaccines, and the mRNA technology has only been in widespread use since late 2020.
The agency is making “huge assumptions” in its consideration of the new Covid boosters, Offit said, adding that it’s possible the new shots may not be any more effective than the existing vaccines.
The data in animals is helpful, he said, but there are pitfalls to solely relying on that kind of data. “The pitfalls are that animals are animals and humans are humans, and although there’s overlap, sometimes there are surprises,” Hotez said.

FDA to authorize new Covid boosters without trials in people
The lack of human data means officials likely won’t know how much better the new shots are — if at all — until the fall booster campaign is well underway.www.nbcnews.com
Are flu vaccines new?
Oh yeah COVID vaccines have been around for decades!!! Dumb assCovid vaccines aren't "new" anymore, either. The technology was evaluated for decades; tested on humans a decade ago with MERs, but never launched when that pandemic didn't spread.
Apparently the testing was just as thorough then as it is now...Covid vaccines aren't "new" anymore, either. The technology was evaluated for decades; tested on humans a decade ago with MERs, but never launched when that pandemic didn't spread.
And here comes Finance85 as Shemp. LOLApparently the testing was just as thorough then as it is now...
Dr. Offit already addressed these questions and is unsatisfied with the reasoning from FDA.Are flu vaccines new? Are they a first time used technology for vaccines?
FIFY.Covid vaccines aren't "new" anymore, either. The technology was evaluated for decades; tested on humans a decade ago with MERs, but never launched because they couldn’t solve the immune-enhancement problem and the technology was deemed too dangerous.
MERs vaccine is >10 years oldOh yeah COVID vaccines have been around for decades!!! Dumb ass
Damn, I was wrong
Apparently the testing was just as thorough then as it is now...
Masks and vaccinations didn't work. How are all those excess deaths going? Still people dying from covid including those vaxxed? You're an idiot.Yeah you are right neither the Iraqi or Afghanistan war worked...that we agree on...but masking and vaccines worked amazingly.
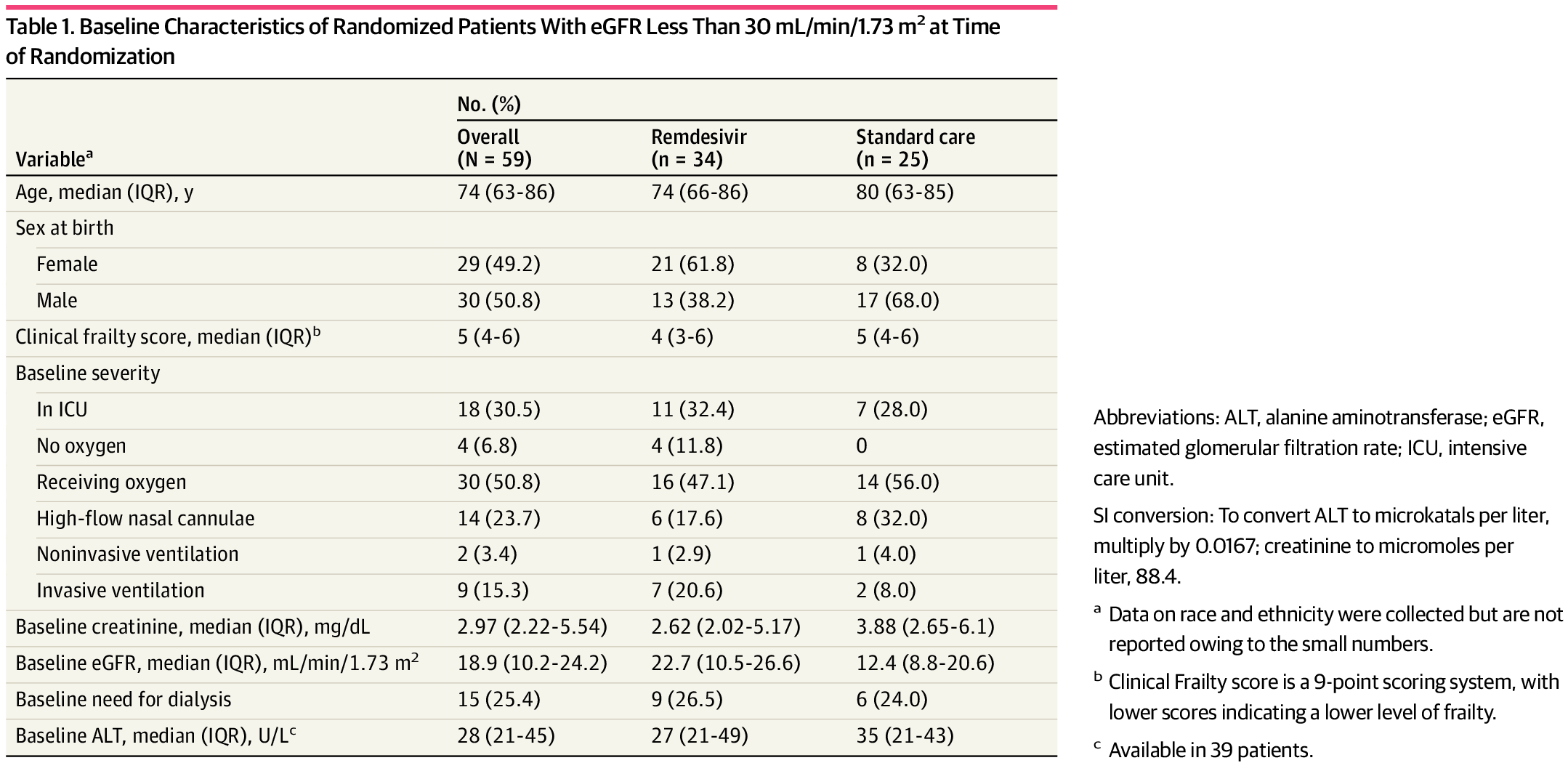
But please keep bringing uo your remdesivir "data" proving how stupid you really are.
Ohh btw you see this new publication? Don't worry it was a rhetorical question as I know you don't read and it hasn't come in tour chain mail yet...

Remdesivir in Patients With Severe Kidney Dysfunction
This secondary analysis of a randomized clinical trial examines the risk of kidney or hepatic toxic effects among patients with impaired kidney function at baseline treated with remdesivir.jamanetwork.com

