FDA Clears the Way for Ridgeback Biotherapeutics to begin Human Testing of a Promising Potential Treatment for COVID-19
4/7/2020
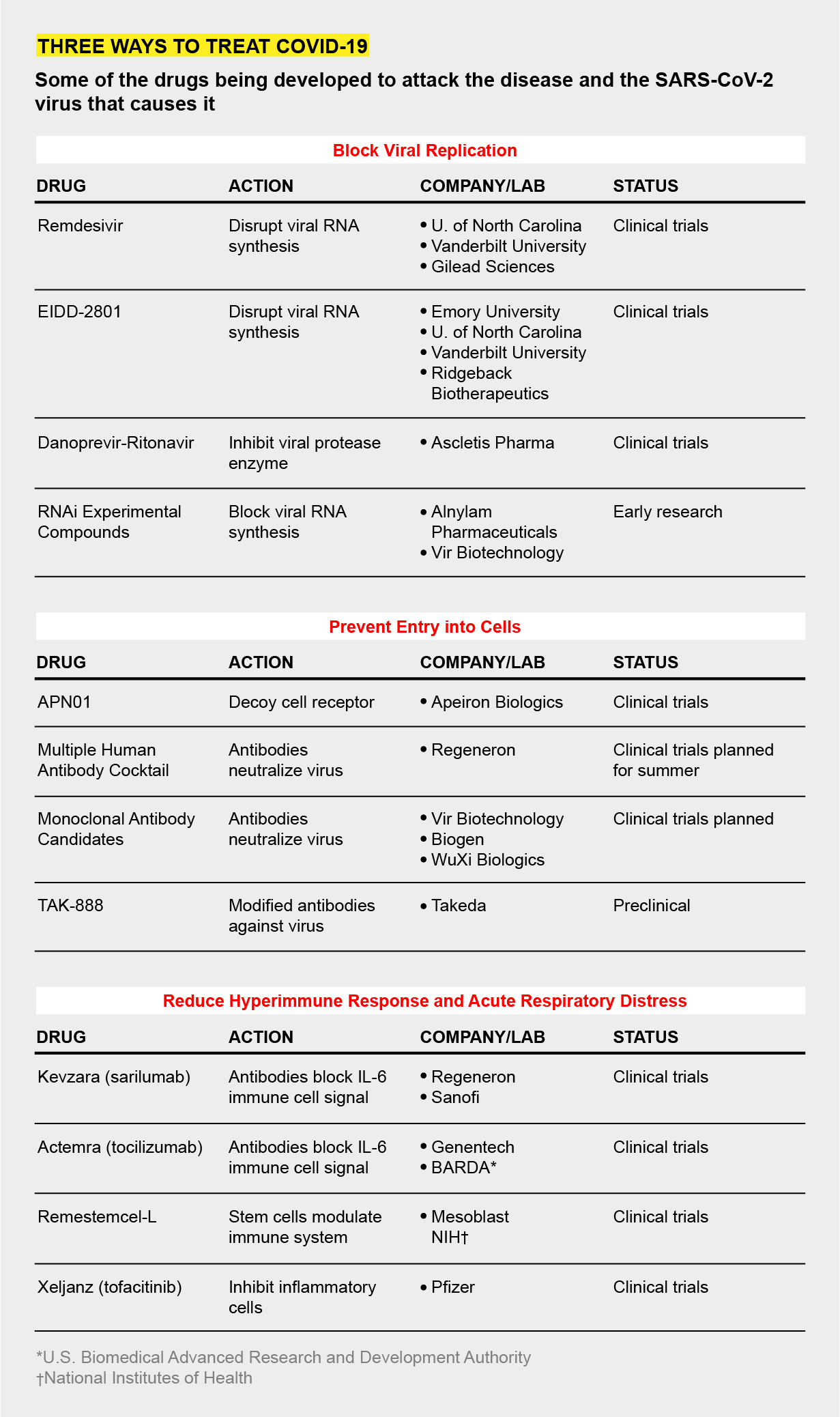
EIDD-2801, an oral broad-spectrum antiviral proceeding into Clinical Trials
ATLANTA and MIAMI, April 6, 2020 /PRNewswire/ -- Ridgeback Biotherapeutics LP, a closely held biotechnology company, and Drug Innovations at Emory (DRIVE), LLC, a not-for-profit biotechnology company wholly owned by Emory University, today announced that the U.S. Food and Drug Administration (FDA) has approved an Investigational New Drug application by Drug Innovation Ventures at Emory (DRIVE), LLC, wholly owned by Emory University, for an orally available antiviral compound, EIDD-2801, exclusively licensed to Ridgeback Biotherapeutics, LP (Ridgeback), a closely held biotechnology company. This action by the FDA allows Ridgeback to initiate human clinical testing of EIDD-2801 in the United States.
EIDD-2801 prevents the replication of SARS-CoV-2, the virus that causes COVID-19, and has shown potent activity against SARS-CoV and MERS-CoV in animal models of infection. In addition to coronaviruses, EIDD-2801 has broad spectrum activity against a number of diseases of public health concern, including influenza, chikungunya, Ebola, and equine encephalitis (VEE and EEE). The antiviral is orally available and, in addition to COVID-19, is being developed for the treatment of seasonal and pandemic influenza under a contract awarded to Emory Institute for Drug Development by the National Institute of Allergy and Infectious Diseases (NIAID) and for Venezuelan and Eastern equine encephalitis virus (VEEV and EEEV) by the Defense Threat Reduction Agency (DTRA).
"FDA's prompt approval of our IND allows us to initiate human testing for EIDD-2801 as quickly as possible," says George Painter, Ph.D., director of the Emory Institute for Drug Development (EIDD) and CEO of DRIVE. "We are grateful to our collaborators for helping us to assemble this application quickly, and to the FDA for expediting the process. An orally available antiviral medication would be a critical weapon for fighting COVID-19."
About EIDD-2801:
EIDD-2801 is an orally bioavailable form of a highly potent ribonucleoside analog that inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19. In animal studies of two distinct coronaviruses (SARS-CoV1 and MERS), EIDD-2801 has been shown to improve pulmonary function, decrease body weight loss and reduce the amount of virus in the lung. In addition to activity against coronaviruses, EIDD-2801, in laboratory studies, has demonstrated activity against seasonal and bird influenza, respiratory syncytial virus, chikungunya virus, Ebola virus, Venezuelan equine encephalitis virus, and Eastern equine encephalitis virus. The development of EIDD-2801 has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), under contract numbers HHSN272201500008C and 75N93019C00058, and from the Defense Threat Reduction Agency (DTRA), under contract numbers HDTRA1-13-C-0072 and HDTRA1-15-C-0075.
https://www.biospace.com/article/re...a-promising-potential-treatment-for-covid-19/
4/7/2020
EIDD-2801, an oral broad-spectrum antiviral proceeding into Clinical Trials
ATLANTA and MIAMI, April 6, 2020 /PRNewswire/ -- Ridgeback Biotherapeutics LP, a closely held biotechnology company, and Drug Innovations at Emory (DRIVE), LLC, a not-for-profit biotechnology company wholly owned by Emory University, today announced that the U.S. Food and Drug Administration (FDA) has approved an Investigational New Drug application by Drug Innovation Ventures at Emory (DRIVE), LLC, wholly owned by Emory University, for an orally available antiviral compound, EIDD-2801, exclusively licensed to Ridgeback Biotherapeutics, LP (Ridgeback), a closely held biotechnology company. This action by the FDA allows Ridgeback to initiate human clinical testing of EIDD-2801 in the United States.
EIDD-2801 prevents the replication of SARS-CoV-2, the virus that causes COVID-19, and has shown potent activity against SARS-CoV and MERS-CoV in animal models of infection. In addition to coronaviruses, EIDD-2801 has broad spectrum activity against a number of diseases of public health concern, including influenza, chikungunya, Ebola, and equine encephalitis (VEE and EEE). The antiviral is orally available and, in addition to COVID-19, is being developed for the treatment of seasonal and pandemic influenza under a contract awarded to Emory Institute for Drug Development by the National Institute of Allergy and Infectious Diseases (NIAID) and for Venezuelan and Eastern equine encephalitis virus (VEEV and EEEV) by the Defense Threat Reduction Agency (DTRA).
"FDA's prompt approval of our IND allows us to initiate human testing for EIDD-2801 as quickly as possible," says George Painter, Ph.D., director of the Emory Institute for Drug Development (EIDD) and CEO of DRIVE. "We are grateful to our collaborators for helping us to assemble this application quickly, and to the FDA for expediting the process. An orally available antiviral medication would be a critical weapon for fighting COVID-19."
About EIDD-2801:
EIDD-2801 is an orally bioavailable form of a highly potent ribonucleoside analog that inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19. In animal studies of two distinct coronaviruses (SARS-CoV1 and MERS), EIDD-2801 has been shown to improve pulmonary function, decrease body weight loss and reduce the amount of virus in the lung. In addition to activity against coronaviruses, EIDD-2801, in laboratory studies, has demonstrated activity against seasonal and bird influenza, respiratory syncytial virus, chikungunya virus, Ebola virus, Venezuelan equine encephalitis virus, and Eastern equine encephalitis virus. The development of EIDD-2801 has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), under contract numbers HHSN272201500008C and 75N93019C00058, and from the Defense Threat Reduction Agency (DTRA), under contract numbers HDTRA1-13-C-0072 and HDTRA1-15-C-0075.
https://www.biospace.com/article/re...a-promising-potential-treatment-for-covid-19/